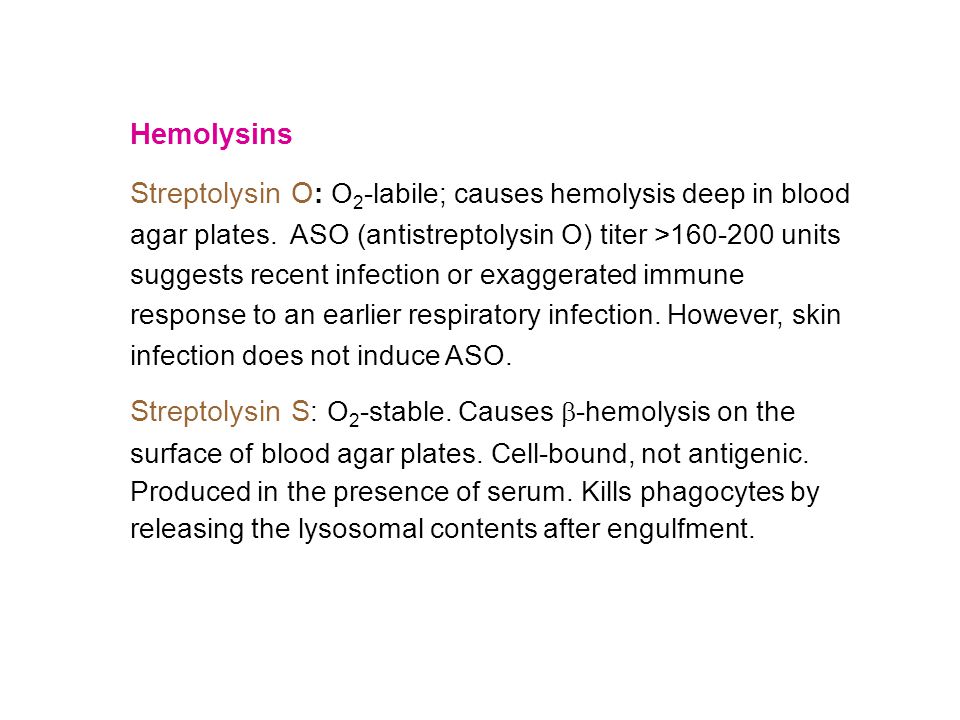
This should be taken into account when performing the assay. The classic hemolytic method for determining the ASO titer was the first test developed to measure what.

Aso And Anti Dnase B Titers In Arf Td And Pharyngitis Patients The Download Scientific Diagram
If positive the antibody concentration is determined by the classical tube test.

Classic hemolytic method aso testing. Cyprotexs in vitro toxic hemolysis assay is a sensitive and accurate method for predicting toxic hemolysis of a drug. This activity can be restored by a reducing agent. The ethics committee of the Tehran University of Medical Sciences approved current study IRTUMSSPHREC1395841.
Between the results ob- tained and those of the classic haemolytic method there was a correlation which could be described by the following equation. ASO test method is based on an immunological reaction between streptococcal exoenzymes bound to biologically inert latex particles and streptococcal antibodies in the test sample. Discard the disposable pipette.
The ASTM F756 Hemolysis procedure was designed to determine the hemolytic properties of the test article. Both methods were used in order to test 591 samples. What was it based on.
The test measures the antioxidants capacity to neutralise the 22-azinobis3- ethylbenzthiazolin-6-sulfonic acid ABTS stable radical cation a blue-green chromophore of maximum absorption at 734 nm whose. More recently various modifications have been published simplifying the performance of the test 5 9 10 16. A new method is proposed for the determination of antistreptoly sin O based on the properties of streptolysin O where the reduced form of the toxin is hemolytic whereas the oxidized form is not.
In this test a standardized solution of streptolysin O is added to tubes containing serial dilutions of the patients serum. At BioAplicada we carry out for our clients the In Vitro Hemolysis Test for medical devices or materials that come into direct or indirect contact with blood. Y 106x 13 r0974 6.
ASO tests have lately been most widely applied in clinical laboratory situations using semiquantitative latex agglutination the hemolysis inhibition method quantitative turbidimetry. Microorganisms that produce toxic materials to mask hemolysis. Todd units or international units.
Serological and Molecular Detection of Bacterial Infections Streptococci This test was based on the ability of patient antibodies to neutralize the hemolytic activity of streptolysin O. Test Information Initial testing consist of direct and indirect antiglobulin tests DAT IAT if the previous testing was non-reactive. Add one drop of ASO Latex reagent next to the drop of serum.
The investigation may include an elution with PEG-IAT antibody identification if required common phenotyping and in rare cases red cell separation or RBC genomic testing if evidence of transfused cells is seen in the phenotyping. However it should be noted that hemolysis sometimes occurs when blood is drawn. Allow each components of the test kit reagents controls to reach room temperature.
A new method is proposed for the determination of antistreptolysin O based on the properties of streptolysin O. The reagent has been adjusted in the way that presence of an ASO titer of 200 IUmL or higher in the serum gives a visible. Based on the ability of patient antibodies to neutralize the hemolytic activity of streptolysin O What is the ASO titer expressed as.
The classic hemolytic method for determining the ASO titer was the first test developed to measure streptococcal antibodies. For presumptive identification of S. Using the flattened end of the appropriate plastic pipette as a stirrer step 2 thoroughly mix each sample with reagent within the full area of the circle.
Read product details online and order your kit today. The classic hemolytic method. Gently shake the latex reagent to disperse the particles.
Hemolysis test were surveyed in the basis of ASTM standard E2524-08 Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles with some modifications in the concentration of analyzed particles 3 4. Sclavo SpA Siena and methods with streptolysin O antigen coated on the surface of biologically inert latex particles. Todd units use streptolysin reagent standards.
The Hemolysis Test is an evaluation of hemocompatibility designed to determine the hemolytic properties of finished medical devices and its components. Determined by the tube dilution hemolysis method of Rantz and Randall the Aso Quantum hemolytic method ISVT. The ASO titer can be expressed in Todd units or international units.
Add one free-falling drop of a reagent to each control and sample. After incubating human group O red blood cells are added. As far as the hemolytic assay is concerned we have attempted to improve the methodology by establishing a new method for the assay of ASO using the patients whole blood and making use of his own erythrocytes to reveal the reaction.
Spurious hemolysis is an important issue in laboratory testing wherein the injury of blood cells especially erythrocytes is associated with release of many intracellular compounds in the surrounding plasma or serum thus introducing a clinically significant bias in test results of many clinical chemistry analytes including glucose 3536. Place a drop of undiluted serum into a circle of a test slide. To increase detection rates after the initial 18 to 24 hours of incubation negative cultures should be re-examined after an additional 24 hours of incubation.
Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles1 This standard is issued under the fixed designation E2524. Hemolytic samples are a rather common and unfavorable occurrence in laboratory practice as they are often considered unsuitable for routine testing. The test is performed using citrated human blood.
All reagents contain 01 Sodium azide as a preservative. Pyogenescultures should be tested for. ASO testing can be done as a screening test by a rapid slide agglutination method.
Mix the ASO latex reagent by gently shaking. The number immediately following the designation indicates the year of original adoption or in the case of revision the year of last revision. Test ability of patient antibodies to neutralize the hemolytic activity of streptolysin O.
What are Todd Units. The procedure involves exposing the test material or material extract to a blood cell suspension and then determining the amount of hemoglobin released. The ASO latex test kit is used for the qualitative and semi-quantitative detection of antibodies.
The TEAC test was first developed by Miller and his team 1993 as a simple and convenient method used to measure the total antioxidant capacity TAC.
Streptococci Ppt Video Online Download
Tidak ada komentar